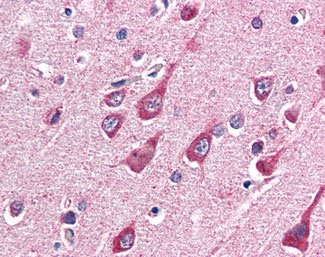
OGT / O-GLCNAC Antibody (Internal)
Goat Polyclonal Antibody
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| WB, IHC-P, E |
|---|---|
| Primary Accession | O15294 |
| Reactivity | Human, Mouse, Rat, Rabbit, Hamster, Monkey, Pig, Horse, Bovine, Dog |
| Host | Goat |
| Clonality | Polyclonal |
| Calculated MW | 117kDa |
| Dilution | ELISA (1:64000), IHC-P (5 µg/ml), WB (1:64000) |
| Gene ID | 8473 |
|---|---|
| Other Names | UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit, 2.4.1.255, O-GlcNAc transferase subunit p110, O-linked N-acetylglucosamine transferase 110 kDa subunit, OGT, OGT |
| Target/Specificity | Human OGT. This antibody is expected to recognise both reported isoforms (NP_858058.1 and NP_858059.1 |
| Reconstitution & Storage | Store at -20°C. Minimize freezing and thawing. |
| Precautions | OGT / O-GLCNAC Antibody (Internal) is for research use only and not for use in diagnostic or therapeutic procedures. |
| Name | OGT {ECO:0000303|PubMed:11773972, ECO:0000312|HGNC:HGNC:8127} |
|---|---|
| Function | Catalyzes the transfer of a single N-acetylglucosamine from UDP-GlcNAc to a serine or threonine residue in cytoplasmic and nuclear proteins resulting in their modification with a beta-linked N- acetylglucosamine (O-GlcNAc) (PubMed:12150998, PubMed:19451179, PubMed:20018868, PubMed:26678539, PubMed:26369908, PubMed:23103939, PubMed:21240259, PubMed:21285374, PubMed:27713473, PubMed:15361863, PubMed:37541260, PubMed:26237509). Glycosylates a large and diverse number of proteins including histone H2B, AKT1, AMPK, ATG4B, CAPRIN1, EZH2, FNIP1, KRT7, LMNA, LMNB1, LMNB2, RPTOR, HOXA1, PFKL, KMT2E/MLL5, MAPT/TAU, TET2, RBL2, RET, NOD2 and HCFC1 (PubMed:19451179, PubMed:20200153, PubMed:21285374, PubMed:22923583, PubMed:23353889, PubMed:24474760, PubMed:26678539, PubMed:26369908, PubMed:27527864, PubMed:30699359, PubMed:34074792, PubMed:34667079, PubMed:37541260, PubMed:26237509). Can regulate their cellular processes via cross-talk between glycosylation and phosphorylation or by affecting proteolytic processing (PubMed:21285374). Involved in insulin resistance in muscle and adipocyte cells via glycosylating insulin signaling components and inhibiting the 'Thr-308' phosphorylation of AKT1, enhancing IRS1 phosphorylation and attenuating insulin signaling (By similarity). Involved in glycolysis regulation by mediating glycosylation of 6- phosphofructokinase PFKL, inhibiting its activity (PubMed:22923583). Plays a key role in chromatin structure by mediating O-GlcNAcylation of 'Ser-112' of histone H2B: recruited to CpG-rich transcription start sites of active genes via its interaction with TET proteins (TET1, TET2 or TET3) (PubMed:22121020, PubMed:23353889). As part of the NSL complex indirectly involved in acetylation of nucleosomal histone H4 on several lysine residues (PubMed:20018852). O-GlcNAcylation of 'Ser-75' of EZH2 increases its stability, and facilitating the formation of H3K27me3 by the PRC2/EED-EZH2 complex (PubMed:24474760). Stabilizes KMT2E/MLL5 by mediating its glycosylation, thereby preventing KMT2E/MLL5 ubiquitination (PubMed:26678539). Regulates circadian oscillation of the clock genes and glucose homeostasis in the liver (By similarity). Stabilizes clock proteins BMAL1 and CLOCK through O-glycosylation, which prevents their ubiquitination and subsequent degradation (By similarity). Promotes the CLOCK-BMAL1-mediated transcription of genes in the negative loop of the circadian clock such as PER1/2 and CRY1/2. O-glycosylates HCFC1 and regulates its proteolytic processing and transcriptional activity (PubMed:21285374, PubMed:28584052, PubMed:28302723). Component of a THAP1/THAP3-HCFC1-OGT complex that is required for the regulation of the transcriptional activity of RRM1 (PubMed:20200153). Regulates mitochondrial motility in neurons by mediating glycosylation of TRAK1 (By similarity). Promotes autophagy by mediating O-glycosylation of ATG4B (PubMed:27527864). Acts as a regulator of mTORC1 signaling by mediating O-glycosylation of RPTOR and FNIP1: O-GlcNAcylation of RPTOR in response to glucose sufficiency promotes activation of the mTORC1 complex (PubMed:30699359, PubMed:37541260). |
| Cellular Location | Nucleus. Cytoplasm. Note=Predominantly localizes to the nucleus (PubMed:26678539). Translocates into the nucleus via association with importin KPNA1 (PubMed:27713473) [Isoform 3]: Cytoplasm. Nucleus. Cell membrane {ECO:0000250|UniProtKB:P56558}. Mitochondrion membrane {ECO:0000250|UniProtKB:P56558}. Cell projection {ECO:0000250|UniProtKB:P56558}. Note=Mostly in the nucleus. Retained in the nucleus via interaction with HCFC1 (PubMed:21285374). After insulin induction, translocated from the nucleus to the cell membrane via phosphatidylinositide binding. Colocalizes with AKT1 at the plasma membrane. TRAK1 recruits this protein to mitochondria. In the absence of TRAK1, localizes in cytosol and nucleus (By similarity) {ECO:0000250|UniProtKB:P56558, ECO:0000269|PubMed:21285374} |
| Tissue Location | Highly expressed in pancreas and to a lesser extent in skeletal muscle, heart, brain and placenta. Present in trace amounts in lung and liver. |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
Catalyzes the transfer of a single N-acetylglucosamine from UDP-GlcNAc to a serine or threonine residue in cytoplasmic and nuclear proteins resulting in their modification with a beta- linked N-acetylglucosamine (O-GlcNAc). Glycosylates a large and diverse number of proteins including histone H2B, AKT1, EZH2, PFKL, KMT2E/MLL5, MAPT/TAU and HCFC1. Can regulate their cellular processes via cross-talk between glycosylation and phosphorylation or by affecting proteolytic processing. Involved in insulin resistance in muscle and adipocyte cells via glycosylating insulin signaling components and inhibiting the 'Thr-308' phosphorylation of AKT1, enhancing IRS1 phosphorylation and attenuating insulin signaling. Involved in glycolysis regulation by mediating glycosylation of 6-phosphofructokinase PFKL, inhibiting its activity (PubMed:22923583). Component of a THAP1/THAP3-HCFC1-OGT complex that is required for the regulation of the transcriptional activity of RRM1. Plays a key role in chromatin structure by mediating O-GlcNAcylation of 'Ser-112' of histone H2B: recruited to CpG-rich transcription start sites of active genes via its interaction with TET proteins (TET1, TET2 or TET3) (PubMed:22121020, PubMed:23353889). As part of the NSL complex indirectly involved in acetylation of nucleosomal histone H4 on several lysine residues (PubMed:20018852). O-GlcNAcylation of 'Ser-75' of EZH2 increases its stability, and facilitating the formation of H3K27me3 by the PRC2/EED-EZH2 complex (PubMed:24474760). Regulates circadian oscillation of the clock genes and glucose homeostasis in the liver. Stabilizes clock proteins ARNTL/BMAL1 and CLOCK through O-glycosylation, which prevents their ubiquitination and subsequent degradation. Promotes the CLOCK-ARNTL/BMAL1-mediated transcription of genes in the negative loop of the circadian clock such as PER1/2 and CRY1/2 (PubMed:12150998, PubMed:18288188, PubMed:19377461, PubMed:19451179, PubMed:20018868, PubMed:20200153, PubMed:21285374, PubMed:15361863).
References
Lubas W.A.,et al.J. Biol. Chem. 272:9316-9324(1997).
Nolte D.,et al.Mamm. Genome 13:62-64(2002).
Bechtel S.,et al.BMC Genomics 8:399-399(2007).
Bienvenut W.V.,et al.Submitted (FEB-2008) to UniProtKB.
Yang X.,et al.Cell 110:69-80(2002).
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.











 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.