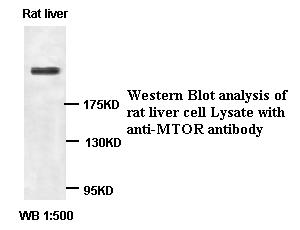
MTOR Antibody
Rabbit Polyclonal Antibody
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| WB, IHC-P |
|---|---|
| Primary Accession | P42345 |
| Other Accession | 2475 |
| Reactivity | Human, Rat |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Calculated MW | 288892 Da |
| Dilution | IHC-P (5 µg/ml), |
| Gene ID | 2475 |
|---|---|
| Other Names | MTOR, FRAP1, FRAP2, FRAP, Mammalian target of rapamycin, RAFT1, Rapamycin and FKBP12 target 1, RAPT1, Rapamycin target protein 1 |
| Reconstitution & Storage | PBS, pH 7.4, 0.02% sodium azide. Store at -20°C for up to one year. |
| Precautions | MTOR Antibody is for research use only and not for use in diagnostic or therapeutic procedures. |
| Name | MTOR (HGNC:3942) |
|---|---|
| Function | Serine/threonine protein kinase which is a central regulator of cellular metabolism, growth and survival in response to hormones, growth factors, nutrients, energy and stress signals (PubMed:12087098, PubMed:12150925, PubMed:12150926, PubMed:12231510, PubMed:12718876, PubMed:14651849, PubMed:15268862, PubMed:15467718, PubMed:15545625, PubMed:15718470, PubMed:18497260, PubMed:18762023, PubMed:18925875, PubMed:20516213, PubMed:20537536, PubMed:21659604, PubMed:23429703, PubMed:23429704, PubMed:25799227, PubMed:26018084, PubMed:29150432, PubMed:31112131, PubMed:31601708, PubMed:32561715, PubMed:34519269, PubMed:29236692, PubMed:37751742). MTOR directly or indirectly regulates the phosphorylation of at least 800 proteins (PubMed:15268862, PubMed:15467718, PubMed:17517883, PubMed:18925875, PubMed:18372248, PubMed:18497260, PubMed:20516213, PubMed:21576368, PubMed:21659604, PubMed:23429704, PubMed:29236692, PubMed:37751742). Functions as part of 2 structurally and functionally distinct signaling complexes mTORC1 and mTORC2 (mTOR complex 1 and 2) (PubMed:15268862, PubMed:15467718, PubMed:18925875, PubMed:18497260, PubMed:20516213, PubMed:21576368, PubMed:21659604, PubMed:23429704). In response to nutrients, growth factors or amino acids, mTORC1 is recruited to the lysosome membrane and promotes protein, lipid and nucleotide synthesis by phosphorylating key regulators of mRNA translation and ribosome synthesis (PubMed:12087098, PubMed:12150925, PubMed:12150926, PubMed:12231510, PubMed:12718876, PubMed:14651849, PubMed:15268862, PubMed:15467718, PubMed:15545625, PubMed:15718470, PubMed:18497260, PubMed:18762023, PubMed:18925875, PubMed:20516213, PubMed:20537536, PubMed:21659604, PubMed:23429703, PubMed:23429704, PubMed:25799227, PubMed:26018084, PubMed:29150432, PubMed:31112131, PubMed:34519269, PubMed:29236692). This includes phosphorylation of EIF4EBP1 and release of its inhibition toward the elongation initiation factor 4E (eiF4E) (PubMed:24403073, PubMed:29236692). Moreover, phosphorylates and activates RPS6KB1 and RPS6KB2 that promote protein synthesis by modulating the activity of their downstream targets including ribosomal protein S6, eukaryotic translation initiation factor EIF4B, and the inhibitor of translation initiation PDCD4 (PubMed:12150925, PubMed:12087098, PubMed:18925875, PubMed:29150432, PubMed:29236692). Stimulates the pyrimidine biosynthesis pathway, both by acute regulation through RPS6KB1-mediated phosphorylation of the biosynthetic enzyme CAD, and delayed regulation, through transcriptional enhancement of the pentose phosphate pathway which produces 5-phosphoribosyl-1- pyrophosphate (PRPP), an allosteric activator of CAD at a later step in synthesis, this function is dependent on the mTORC1 complex (PubMed:23429704, PubMed:23429703). Regulates ribosome synthesis by activating RNA polymerase III-dependent transcription through phosphorylation and inhibition of MAF1 an RNA polymerase III-repressor (PubMed:20516213). Activates dormant ribosomes by mediating phosphorylation of SERBP1, leading to SERBP1 inactivation and reactivation of translation (PubMed:36691768). In parallel to protein synthesis, also regulates lipid synthesis through SREBF1/SREBP1 and LPIN1 (By similarity). To maintain energy homeostasis mTORC1 may also regulate mitochondrial biogenesis through regulation of PPARGC1A (By similarity). In the same time, mTORC1 inhibits catabolic pathways: negatively regulates autophagy through phosphorylation of ULK1 (PubMed:32561715). Under nutrient sufficiency, phosphorylates ULK1 at 'Ser-758', disrupting the interaction with AMPK and preventing activation of ULK1 (PubMed:32561715). Also prevents autophagy through phosphorylation of the autophagy inhibitor DAP (PubMed:20537536). Also prevents autophagy by phosphorylating RUBCNL/Pacer under nutrient-rich conditions (PubMed:30704899). Prevents autophagy by mediating phosphorylation of AMBRA1, thereby inhibiting AMBRA1 ability to mediate ubiquitination of ULK1 and interaction between AMBRA1 and PPP2CA (PubMed:23524951, PubMed:25438055). mTORC1 exerts a feedback control on upstream growth factor signaling that includes phosphorylation and activation of GRB10 a INSR-dependent signaling suppressor (PubMed:21659604). Among other potential targets mTORC1 may phosphorylate CLIP1 and regulate microtubules (PubMed:12231510). The mTORC1 complex is inhibited in response to starvation and amino acid depletion (PubMed:12150925, PubMed:12150926, PubMed:24403073). The non- canonical mTORC1 complex, which acts independently of RHEB, specifically mediates phosphorylation of MiT/TFE factors MITF, TFEB and TFE3 in the presence of nutrients, promoting their cytosolic retention and inactivation (PubMed:22576015, PubMed:22343943, PubMed:22692423, PubMed:24448649, PubMed:32612235, PubMed:36608670, PubMed:36697823). Upon starvation or lysosomal stress, inhibition of mTORC1 induces dephosphorylation and nuclear translocation of TFEB and TFE3, promoting their transcription factor activity (PubMed:22576015, PubMed:22343943, PubMed:22692423, PubMed:24448649, PubMed:32612235, PubMed:36608670). The mTORC1 complex regulates pyroptosis in macrophages by promoting GSDMD oligomerization (PubMed:34289345). MTOR phosphorylates RPTOR which in turn inhibits mTORC1 (By similarity). As part of the mTORC2 complex MTOR may regulate other cellular processes including survival and organization of the cytoskeleton (PubMed:15268862, PubMed:15467718). mTORC2 plays a critical role in the phosphorylation at 'Ser-473' of AKT1, a pro-survival effector of phosphoinositide 3- kinase, facilitating its activation by PDK1 (PubMed:15718470). mTORC2 may regulate the actin cytoskeleton, through phosphorylation of PRKCA, PXN and activation of the Rho-type guanine nucleotide exchange factors RHOA and RAC1A or RAC1B (PubMed:15268862). mTORC2 also regulates the phosphorylation of SGK1 at 'Ser-422' (PubMed:18925875). Regulates osteoclastogenesis by adjusting the expression of CEBPB isoforms (By similarity). Plays an important regulatory role in the circadian clock function; regulates period length and rhythm amplitude of the suprachiasmatic nucleus (SCN) and liver clocks (By similarity). |
| Cellular Location | Lysosome membrane; Peripheral membrane protein; Cytoplasmic side Endoplasmic reticulum membrane; Peripheral membrane protein; Cytoplasmic side. Golgi apparatus membrane; Peripheral membrane protein; Cytoplasmic side. Mitochondrion outer membrane; Peripheral membrane protein; Cytoplasmic side. Cytoplasm. Nucleus {ECO:0000250|UniProtKB:Q9JLN9}. Nucleus, PML body {ECO:0000250|UniProtKB:Q9JLN9}. Microsome membrane. Cytoplasmic vesicle, phagosome. Note=Shuttles between cytoplasm and nucleus. Accumulates in the nucleus in response to hypoxia (By similarity). Targeting to lysosomes depends on amino acid availability and RRAGA and RRAGB (PubMed:18497260, PubMed:20381137). Lysosome targeting also depends on interaction with MEAK7. Translocates to the lysosome membrane in the presence of TM4SF5 (PubMed:30956113) {ECO:0000250|UniProtKB:Q9JLN9, ECO:0000269|PubMed:18497260, ECO:0000269|PubMed:20381137, ECO:0000269|PubMed:29750193, ECO:0000269|PubMed:30956113} |
| Tissue Location | Expressed in numerous tissues, with highest levels in testis. |
| Volume | 50 µl |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
Serine/threonine protein kinase which is a central regulator of cellular metabolism, growth and survival in response to hormones, growth factors, nutrients, energy and stress signals. MTOR directly or indirectly regulates the phosphorylation of at least 800 proteins. Functions as part of 2 structurally and functionally distinct signaling complexes mTORC1 and mTORC2 (mTOR complex 1 and 2). Activated mTORC1 up-regulates protein synthesis by phosphorylating key regulators of mRNA translation and ribosome synthesis. This includes phosphorylation of EIF4EBP1 and release of its inhibition toward the elongation initiation factor 4E (eiF4E). Moreover, phosphorylates and activates RPS6KB1 and RPS6KB2 that promote protein synthesis by modulating the activity of their downstream targets including ribosomal protein S6, eukaryotic translation initiation factor EIF4B, and the inhibitor of translation initiation PDCD4. Stimulates the pyrimidine biosynthesis pathway, both by acute regulation through RPS6KB1- mediated phosphorylation of the biosynthetic enzyme CAD, and delayed regulation, through transcriptional enhancement of the pentose phosphate pathway which produces 5-phosphoribosyl-1- pyrophosphate (PRPP), an allosteric activator of CAD at a later step in synthesis, this function is dependent on the mTORC1 complex. Regulates ribosome synthesis by activating RNA polymerase III-dependent transcription through phosphorylation and inhibition of MAF1 an RNA polymerase III-repressor. In parallel to protein synthesis, also regulates lipid synthesis through SREBF1/SREBP1 and LPIN1. To maintain energy homeostasis mTORC1 may also regulate mitochondrial biogenesis through regulation of PPARGC1A. mTORC1 also negatively regulates autophagy through phosphorylation of ULK1. Under nutrient sufficiency, phosphorylates ULK1 at 'Ser- 758', disrupting the interaction with AMPK and preventing activation of ULK1. Also prevents autophagy through phosphorylation of the autophagy inhibitor DAP. mTORC1 exerts a feedback control on upstream growth factor signaling that includes phosphorylation and activation of GRB10 a INSR-dependent signaling suppressor. Among other potential targets mTORC1 may phosphorylate CLIP1 and regulate microtubules. As part of the mTORC2 complex MTOR may regulate other cellular processes including survival and organization of the cytoskeleton. Plays a critical role in the phosphorylation at 'Ser-473' of AKT1, a pro-survival effector of phosphoinositide 3-kinase, facilitating its activation by PDK1. mTORC2 may regulate the actin cytoskeleton, through phosphorylation of PRKCA, PXN and activation of the Rho-type guanine nucleotide exchange factors RHOA and RAC1A or RAC1B. mTORC2 also regulates the phosphorylation of SGK1 at 'Ser-422'.
References
Brown E.J.,et al.Nature 369:756-758(1994).
Onyango P.,et al.Genomics 50:187-198(1998).
Nakajima D.,et al.Submitted (MAR-2005) to the EMBL/GenBank/DDBJ databases.
Gregory S.G.,et al.Nature 441:315-321(2006).
Stover C.,et al.Genes Immun. 2:119-127(2001).
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.











 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.