Pro-MMP-2, mouse recombinant protein
Matrix metalloproteinase-2, MMP, MMP recombinant, MMP protein, MMP inhibitor
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND
| Primary Accession | Q3UG07 |
|---|---|
| Concentration | 0.05 mg/ ml; >40 mU/mg (Masui substrate), >40 mU/mg (Knight substrate) - APMA activation, 1 hour/37oC |
| Calculated MW | 40/42 kDa |
| Gene ID | 17390 |
|---|---|
| Gene Symbol | MMP2 |
| Other Names | 72 kDa type IV collagenase (EC 3.4.24.24) (72 kDa gelatinase) (Gelatinase A) (Matrix metalloproteinase-2) (MMP-2) [Cleaved into: PEX] |
| Gene Source | Human |
| Source | mouse fibroblasts |
| Assay&Purity | SDS-PAGE; ≥90% |
| Assay2&Purity2 | HPLC; |
| Recombinant | No |
| Format | Liquid |
| Storage | -80°C; In 50 mM Tris-HCl, pH 7; 200 mM NaCl; 5 mM CaCl₂; 1 µM ZnCl₂; 0.05% Brij 35; 0,05% NaN₃ |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
The progelatinase A, a member of the matrix metalloproteinase (MMP) family, has been isolated from macrophages and fibroblasts. Gelatinase A hydrolyses several components of the extracellular matrix, e.g. the collagen types IV, V and XI and gelatin. Progelatinase A complexed via their C-terminal domain with TIMP-2 was isolated from culture media of different cell types. This complex shows both properties of its constituents: Like TIMP-2 it inhibits active matrix metalloproteinases and like gelatinase it shows proteolytic activity after activation with APMA (4-aminophenylmercury acetate). However, its proteolytic activity is less than 10% of that of gelatinase A not complexed with TIMP-2. In contrast to the other MMPs the progelatinase A cannot be activated by the serine proteinase trypsin. Until quite recently a potential natural activator that can transform latent progelatinase A into the active form was unknown. It was shown that the catalytic domain of the membrane type 2-matrix metalloproteinase activates progelatinase A as well as the progelatinase A / TIMP-2 complex, by cleaving the 72 kDa progelatinase A to yield 67 kDa gelatinase A, which is than transformed into 62 kDa gelatinase A. The 62 kDa form is about twice as active as the 67 kDa form towards the Dnp-pepitde (Masui et al.). No significant difference in activity was found between free and complexed gelatinase A forms.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.




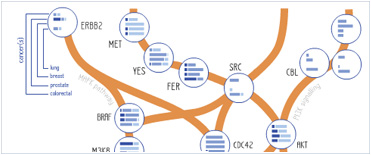







 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.

